Glass is highly resistant to many chemicals, including aqueous solutions, organic substances, halogens such as chlorine and bromine, alkali solutions and most acids. However, some chemicals like hydrofluoric acid, phosphoric acid and hot concentrated alkali solutions can still corrode glass.
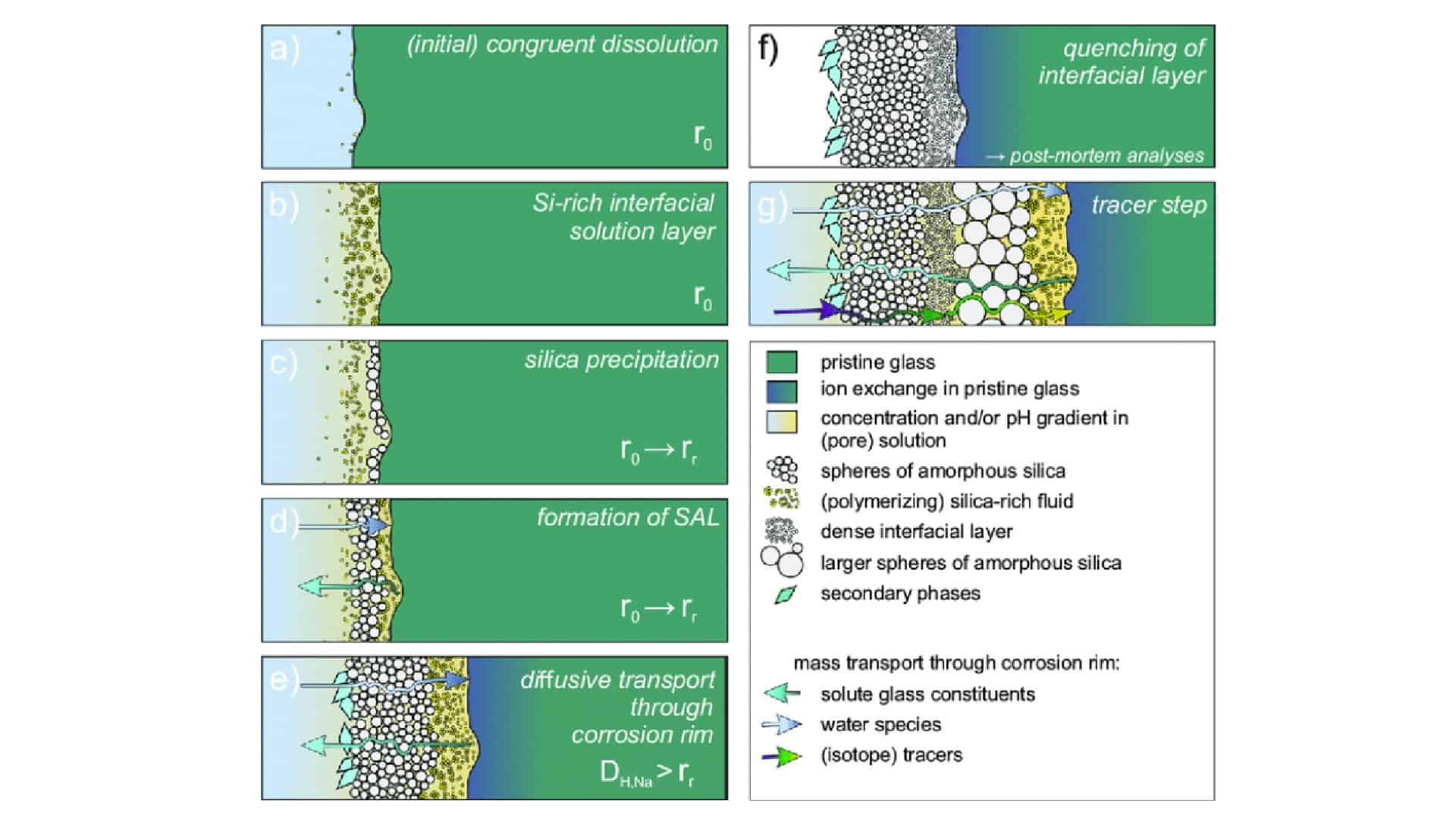
Monte Carlo simulations reveal that glass’ corrosion behavior is determined by a combination of its structure and properties, the valence state of multivalent ions, and the solution in contact with it. Simulations demonstrate three stages in borosilicate glass corrosion – each with distinct kinetic rates.
Hydrofluoric Acid
Hydrofluoric acid (HF) is a chemical commonly used in various industries for rust removal. It also supplies fluorine, the basis for many pharmaceuticals and polymers like Teflon. Unfortunately, HF has an acidic nature which makes it highly corrosive; HF can easily dissolve glass it comes into contact with.
Hydrofluoric acid (HF) is usually not a problem for most glass materials, such as borosilicate or fused silica, commonly used in laboratory instruments. However, when exposed to excessive amounts of HF at elevated temperatures in solution, some material damage may occur.
Some nickel alloys are particularly resistant to hydrogen fluoride at high temperatures and may be suitable for applications where this property is necessary. For instance, Ni-Cu alloy 400 (UNS N04400) has been tested successfully in both aqueous and hydrogen fluoride environments.
It is essential to remember that HF is toxic and should never be used without taking appropriate safety precautions. It can absorb through the skin or into the eyes, leading to severe tissue damage as well as difficulty breathing properly.
Furthermore, HF can damage the lungs if inhaled and is toxic to the kidneys. Prolonged exposure may lead to fluorosis – a condition characterized by yellow teeth, leukopenia, anemia and bone loss – as well as other health effects.
Hydrochloric Acid
Glass is remarkably resistant to acids due to its stable atomic structure that makes it inert against most acids. Only certain acids, especially those containing fluorine, can dissolve good german glass sufficiently.
Glass dissolves at the atomic level, when chemical bonds between individual atoms are broken. These bonds, known as hydroxyls and hydrocarbons, are called hydroxyls and hydrocarbons. Acids based on fluorine can easily break these hydroxyl and hydrocarbon bonds, dissolving glass.
Borosilicate glass is inherently acid-resistant, with the exception of hot concentrated phosphoric acid and hydrofluoric acid. This resistance comes from its high percentage of silica (SiO2) content in borosilicate glass.
Furthermore, this material is highly resistant to thermal shock. This property helps ensure safe operations of laboratory equipment.
However, it is essential to remember that no laboratory test can guarantee accurate service behavior under all conditions. Many factors influence corrosion rate such as solution concentration, agitation speed and other chemicals present in the system.
Sulfuric Acid
Sulfuric acid (H2SO4) is an extremely strong and corrosive liquid used to make fertilizers, explosives, other acids, glue, pickling metal surfaces to remove rust or other contaminants.
It is a highly toxic substance and should be handled with extreme caution. This is due to its potential to cause chemical and thermal burns as well as decompose proteins and lipids, potentially leading to permanent blindness if it comes into contact with eyes.
The most serious danger associated with sulfuric acid lies in skin exposure. Not only can splashing the acid cause chemical and thermal burns, but ingestion of it may cause severe internal organ damage as well.
Another potential risk is inhalation of aerosols which can cause serious eye, respiratory tract and mucous membrane irritation. That’s why using proper PPE when handling this corrosive material is paramount.
Glass is naturally resistant to most acids due to its strong and stable atomic structure. Therefore, when german glass dissolves in an acid, this only happens through chemical reaction.
Glass is highly resistant to corrosion unless the acid used is concentrated. Most corrosives are weaker than hydrofluoric, phosphoric or sulphuric acid and thus require extensive time and effort for effectivity.
Chloride Acids
Glass is resistant to a wide range of chemicals, such as saline solutions, organic substances and halogens like chlorine or bromine. Unfortunately, there are some corrosives which may cause corrosion or dissolve glass under certain circumstances.
Acids, especially hydrofluoric acid and concentrated phosphoric acids, can quickly damage silicate glass due to their higher destructive power than other types of acids. Temperature also plays an important role in this process for greater damage to silicate glass than other acids.
Chloride acids are chemical compounds in which the -OH group has been replaced by a chlorine atom. While there are various such compounds, they all share one characteristic: the chlorine atom is more active than its counterpart in the acid.
Acyl chlorides (also referred to as acid chlorides) are a type of chemical compound formed when carboxylic acids react with thionyl chloride to form a chlorosulfite intermediate.
These compounds are highly hazardous and should be handled with extreme caution. Furthermore, they have lachrymatory properties – meaning they react with water on the surface of the eye to produce hydrochloric and organic acids which cause irritation to eyes.
Borosilicate glass has a much greater chemical resistance than other glasses of similar properties, particularly to hydrofluoric acid, concentrated phosphoric acid and strong caustic solutions at elevated temperatures. Furthermore, this type of glass can handle most organic acids and alkalis with ease.
Alkalis
Alkaloids are compounds with a pH greater than 7. These include alkali metals such as sodium hydroxide (NaOH) and potassium hydroxide (KOH).
They can be obtained naturally in sources like sodium nitrate or industrially from soda ash and caustic soda. They play an essential role in the production of many products like glass, soap, detergents, textiles, and water softeners.
These chemicals are highly corrosive and cause severe burns when in contact with tissues like the mouth or throat. Although they have medical applications to treat diseases, their widespread usage and potential toxicities when used over an extended period of time must be taken into consideration.
Acid corrosion and alkali corrosion both attack silica in glass compositions in distinct ways. When acid attacks the surface, it dissolves the silica layer and exposes a new one – this process is known as ion exchange – increasing surface area for attack by corrosion solutions.
Once in solution, ions in the solution compete to replace those present in glass. Provided there is enough alkali supply, corrosion will proceed at a steady pace.
Alkali solutions can cause microscopic damage to glass fibers, but it can be severe enough to lead to cracking and pitting of the fibers as well as interfacial deterioration between them and resin [43]. This deterioration tends to be more severe than acidic environments and affect both strength and modulus significantly [45].
Water
Water is an odorless, colorless substance that covers approximately three quarters of Earth’s surface in both solid and liquid form. Additionally, it appears in the lower atmosphere as water vapor.
Aquatic solutions are ideal solvents for many substances due to their polar structure – composed of molecules with hydrogen and oxygen atoms.
Water’s polar structure causes it to be attracted to many different molecules, including salt. When water comes into contact with a salt molecule, however, the attractive forces between them are disrupted and the particles dissolve together.
Corrosion in water can be caused by a number of factors. These include the water’s chemical and physical characteristics (temperature, gases, and solid particles), as well as any salts or chemicals dissolved there.
These factors can accelerate the corrosion of metal pipes if exposed to electrochemical potentials. This may occur if exposed to stray electric currents or soil that transfers ions from one location to another.
Fortunately, product teams can keep corrosion to a minimum with certain materials. These include silane, polypropylene (PP), and vinyl ester. These substances provide an effective barrier against aggressive chemicals while still allowing the materials they protect to function normally.
